Protecting Patient Privacy with RFID Technology
By: Shaun Stigall

Company Profile
AUM Cardiovascular, a private company based in Northfield, Minnesota, is a leader in the development of non-invasive, acoustic and ECG-based systems for the assessment of cardiovascular disease.
Business Situation
Heart disease is the leading cause of death for both men and women in the U.S. (approximately 23.4%) and is also the leading cause of death worldwide. Yet, most cardiovascular diagnostic tools are accompanied by a hefty price tag, with nuclear stress tests averaging $13,000 per test and echocardiograms cost approximately $600 each. In contrast, CADence,™ a non-invasive handheld acoustic and ECG device that helps physicians detect physiological and pathological heart murmurs, carries a retail cost of around $50 per test. Using RFID technology, the radiation-free HIPPA-compliant tool effectively protects privacy by de-identifying sensitive information stored in single-use patient booklets.
Technical Situation
The CADence System comprises a digital stethoscope to record cardiac sounds, integrated sensors to record electrical activity of the heart (ECG), a single-use patient booklet and the CADence Software application. Protecting patient privacy is a primary concern for AUM Cardiovascular. Working directly with hospitals and Identiv, AUM Cardiovascular embedded the Identiv RFID Intelligent Inlay and ICODE SLIX IC by NXP Semiconductors into single-use patient booklets, which are scanned by an RFID reading circuit in the handheld device. In addition to protecting privacy by de-identifying the patient, the RFID-enabled booklets provide real-world utilization data and alert AUM Cardiovascular when customers are due to order more tests.
Solution
AUM Cardiovascular sought a collaborative partner to engineer the RFID inlay embedded in its one-time use patient booklets – and ensure that it communicates properly with the RFID reading circuit in the handheld device. Identiv is an expert in designing and manufacturing high-frequency (HF) and UHF transponders for embedded use in everyday objects, including medical devices, books, toys and athletic apparel. Identiv’s transponders can be delivered in a range of form factors, including labels (printed or not), dry inlays, wet inlays (with backing adhesive), tickets and more.
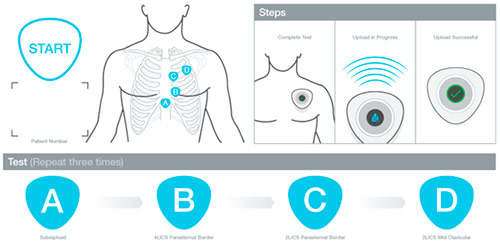
For its one-time CADence patient booklets, AUM Cardiovascular chose Identiv’s 116 mm (4.57 in) RFID label, which is made of white matte paper (thermal transfer printable) and specialized substrate. The 13.56 MHz frequency label is fully compliant with industry standards ISO/IEC 15693, 18000-3 and 28560 – and is optimized to deliver maximum performance in the customer’s environment.
Benefits
By selecting Identiv as a partner to help create CADence, AUM Cardiovascular developed a non-invasive handheld acoustic and ECG device that allows physicians to detect physiological and pathological heart murmurs at a low price point. AUM Cardiovascular chose Identiv because of the company’s RFID engineering skills and experience in designing customized solutions. CADence – which saves hospitals both time and money – uses Identiv RFID technology to protect privacy by de-identifying patient information stored and read in one-time booklets. The complete solution offers clinicians a secure and medically proven method of spotting heart disease before it’s too late.
Best RFID Health Care Implementation 2018 at RFID Journal LIVE!
AUM Cardiovascular was selected as a finalist in the category of Best RFID Health Care Implementation 2018. AUM Cardio will present at RFID Journal LIVE!, which takes place April 10-12 at the Orange County Convention Center, in Orlando, FL. The award sessions are from 10:30 AM to 11:30 AM on April 12. The winners will be announced in the Awards Theater in the exhibit hall at 1 PM on April 12. AUM Cardio will also be on-hand to give live demos in Identiv booth #1038 during the show.
Have an RFID Project?
Contact Identiv to see how we can help turn your project into a reality. Please fill out the Contact Sales form at the bottom of the RFID Inlays product page and a rep will get back to you.